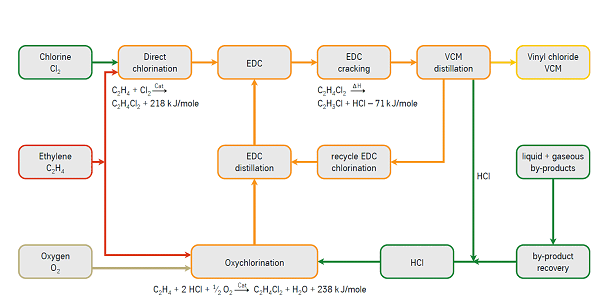
EDC/VCM-Process
The Vinnolit process for the production of vinyl chloride monomer (VCM) from ethylene and chlorine proceeds via two different routes.
In the DIRECT CHLORINATION as well as in the OXYCHLORINATION process, ethylene-dichloride (EDC) is produced. Both reactions proceed exothermally.
EDC produced by direct chlorination can be sent directly to cracking unit whereas the EDC from the oxychlorination process has to pass a purification stage (EDC DISTILLATION) before cracking.
In the EDC CRACKING unit, EDC is cracked to VCM and hydrogen chloride. These, together with any unconverted EDC, are separated in a VCM DISTILLATION unit. The VCM, as final product, is sent to the next production stage or for dispatch. The HCl is returned to the OXYCHLORINATION unit and the unconverted EDC via EDC DISTILLATION unit to the CRACKING section.
DIRECT CHLORINATION: C2H4 + Cl2 ——> C2H4Cl2 + 218 kJ/mole
OXYCHLORINATION: C2H4 + 2 HCl + ½ O2 ——> C2H4Cl2 + H2O + 238kJ/mole
EDC CRACKING: C2H4Cl2 ——> C2H3Cl + HCl – 71 kJ/mole
Water obtained from OXYCHLORINATION process is stripped free of chlorinated hydrocarbons and treated in appropriate water purification plants. Waste gases as well as liquid by-products are fed to the HCl RECOVERY unit and converted to HCl.
The recovered HCl is re-used either for the production of hydrochloric acid or in the OXYCHLORINATION process. This leads to a complete usage of the chlorine input.
The modern Vinnolit processes for the production of VCM have the following distinctive features:
Energy efficient:
- Energy integration with caustic soda plant possible
- Energy integration with PVC plant possible
- Generation of steam in the EDC Distillation
- Production of furnace feed EDC in the Direct Chlorination without distillation
The modern Vinnolit processes for the production of VCM have the following distinctive features:
Energy efficient:
- Reliable reaction control
- Process-proven materials and equipment
- State-of-the-art process control system
The modern Vinnolit processes for the production of VCM have the following distinctive features:
Energy efficient:
- Low energy consumption due to the utilization of reaction and waste gas heat and integration with PVC or caustic soda plants
- High yields
- Optimised reaction conditions and reaction control
- Utilisation of by-products containing Cl2 along with recycling of HCl gas
- High on stream factor
- Low personnel requirements
- Low maintenance costs
- High flexibility, wide range of load
Low consumption figures:
Expected consumption figures for raw materials
and utilities per 1,000 kg VCM product
- Ethylene (100 %) 459 kg
- Chlorine (100 %) 575 kg
- Oxygen..(100 %) 139 kg
- Steam 250 kg 1)
- Fuel gas 2,700,000 kJ
- Combined figure for PVC and VCM plant: 435 kg ....(Energy will be exported to PVC)
